Ciprofloxacin is a synthetic antibacterial agent. It belongs to the fluoroquinolone family of biologically active agents. Its antibacterial activity can be ascribed to its ability to alter bacterial deoxyribonucleic acid (DNA) gyrase. It is a second-generation fruoroquinolone. Chemically it is known as 1-cyclopropyl-6-fluoro-1, 4-dihydro-4-oxo-7-(1-piperazinyl)-3-quinolinecarboxylic acid. It is commonly marketed as Ciflox; Cipro; Ciprobay; Ciproxin; Ciproxine; Plenolyt; Septocipro. Other brand names may exist in different markets.
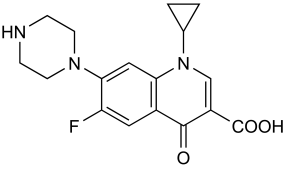
Structural Formula
The empirical formula of ciprofloxacin is C17H18FN3O3 and its structural formula is as follows:
Stereoisomerism
Compounds which differ only by the arrangement in space of the constituent atoms and groups are known as Stereoisomers. There are two groups of stereoisomers; enantiomers and diastereoisomers. enantiomers are are compounds which differ only in the spatial arrangement of their constituent atoms or groups and may be classified into two groups, namely enantiomers and diastereoisomers. enantiomers are substances whose structure is the mirror image of each other. For this reason enantiomers have similar energy. Two enantiomers would therefore have same physichochemical properties. Stereoisomerism may affect the biological activity of a substance.
Clinical Uses
Ciprofloxacin has been to treat many bacterial infections. In some cases it is effective when used alone while in other cases it must be combined with another agent to achieve the desired effect. Emerging resistance to this agent has been reported.
Bone and Joint Infections
Ciprofloxacin has been used to treat bone and joint infections caused by sensitive organisms (pseudomonas aeruginosa, Enterobacter cloacae Serratia marcescens, Klebsiella pneumonia, Proteus mirabilis, Escherichia coli, Staphylococcus aureus, and methicin resistant staphylococci) (Young 1987).
Endocarditis
Ciprofloxacin may also be used to treat prosthetic valve infections caused by Haemophilus aphrophilus, H. influenzae, H. parainfluenzae, H. paraphrophilus, and uncomplicated staphylococcus aureus valve infections (Baddour et al. 2005).
GI Infections
It is active against organisms causing infectious diarrhea including E. coli, Campylobacter of the subspecies. jejuni, Salmonella, Shigella flexneri, S. boydii, S. sonnei, S. dysenteriae, and Vibrio cholerae.
Meningitis and CNS Infections
It has some activity against organisms (Ps. aeruginosa, Salmonella, Neisseria meningitidis) causing CNS infections. However, its safety is questionable. It also been used to treat otitis caused by Ps. aeruginosa (Traub 1987).
Respiratory Tract Infections
Susceptible organisms causing respiratory infections include E. cloacae, E. coli, Haemophilus influenzae, H. parainfluenzae, K. pneumoniae, P. mirabilis, Ps. aeruginosa, S. pneumoniae, S. aureus, Moraxella catarrhalis, and S. pneumonia (Lagast 1985)
Skin Infections
It can be used to treat skin infections caused by C. freundii, E. cloacae, E. coli, K. oxytoca, K. pneumoniae, M. morganii, P. mirabilis, P. vulgaris, P. stuartii, Ps. aeruginosa,S. marcescens, S. aureus, S. epidermidis, and S. pyogenes (Hirschhorn 1987).
Urinary Tract Infections (UTIs)
Ciprofloxacin is used for the treatment of urinary tract infections in adults. It is not favored in pediatric cases because there is an increased risk for adverse outcomes. The following organisms are sensitive to ciprofloxacin: E. coli, P. mirabilis, S. saprophyticus, or E. faecalis, Citrobacter diversus, C. freundii, E. cloacae, K. pneumoniae, M. morganii, P. mirabilis, Providencia rettgeri, Ps. aeruginosa, S. marcescens, E. aerogenes Klebsiella oxytoca, P. stuartii, S. aureus, S. epidermidis, Haemophilus ducreyi, Neisseria gonorrhoeae, and Klebsiella granulomatis (Hoepelman 1987).
Others
Other organisms for which ciprofloxacin is active include Bacillus anthracis, Bartonella henselae, Brucella melitensis, Mycobacterium tuberculosis,Yersinia pestis, plasmodium faciparum, Francisella tularensis, and Ricketssia spp. it must be used in combination with other drugs in the treatment of P. falciparum infections (Ligtvoet1985). Ciprofloxacin is highly active against anthrax and has been used as the drug of choice for this infection in some countries.
There is an emerging resistance to this agent. Reports have largely come from the developing countries where drug therapy is thought to be interrupted before a full dose has been taken.
Mechanism of Action
Ciprofloxacin is active against a wide range of bacteria. Its activity covers both Gram-negative and Gram-positive bacteria. It inhibits bacterial DNA gyrase that cleaves bacterial DNA during replication (smith 1986).
Side Effects
Ciprofloxacin is relatively safe. In most instances, its side effects are mild (Piddock1987). However, serious adverse reactions may occur. Children and the elderly patients are more likely to experience the adverse reactions as compared to other patients receiving the same therapy. Before administering this agent the status of the liver and kidney should be checked. This is because these two organs are major sites of metabolism and excretion. Side effects that may occur after ciprofloxacin therapy are:
- irreversible peripheral neuropathy
- spontaneous tendon rupture and tendonitis
- acute liver failure and hepatitis may occur together or separately
- torsades de pointes- prolongation of the QT segment of the electrocardiogram (Hawkey 1984).
- toxic epidermal necrolysis (TEN)- this can be very severe in some cases.
- Stevens–Johnson syndrome,
- severe central nervous system disorders (CNS) -Psychotic reactions and confusional states, drug induced psychosis, and chorea (Turgeon 1987).
- Clostridium difficile associated disease; pseudomembranous colitis
- altered vision- irreversible loss of vision, impaired color vision as well as photosensitivity reactions. These reactions may be manifested by corneal perforation, evisceration and enucleation.
- acute pancreatitis
- bone marrow depression- this can lead to other complications. The complications may include neutropenia and leucopenia.
- interstitial nephritis
- hemolytic anemia
- exanthema
- abdominal pain
- malaise
- drug fever
- dysaesthesia
- eosinophilia
- Pseudotumor cerebri- increased intracranial pressure causing intracranial hypertension.
Drug Interactions for Ciprofloxacin
Ciprofloxacin can interact with other pharmacological agents in the body and tissues. This interaction can result in either an increase or a decrease of the bioavalability of ciprofloxacin or the interacting drug. Sometimes the concentration of either agent may rise to dangerous levels. Therefore, they should be used with caution when other drugs are already being administered.
- Aminoglycosides- thought to have additive antibacterial effects against Enterobacteriaceae and Ps. aeruginosa
- Antacids (aluminum-, magnesium-, or calcium-containing)- cause decreased absorption of ciprofloxacin.
- Anticoagulants, oral (warfarin) – may enhance warfarin effects. Should be used cautiously. Use coagulation tests to monitor the effects.
- Antimuscarinics (scopolamine, pirenzepine) – thought to prolong absorption of ciprofloxacin in the intestinal tract.
- Bismuth- Enterobacteriaceae.
- Subsalicylate- may cause a slight decrease in the peak plasma concentrations of ciprofloxacin. However, these effects are not clinically important.
- Caffeine- Possible prolonged half-life of caffeine- Advice patients receiving ciprofloxacin to avoid regular consumption of large quantities of coffee, tea, or caffeine-containing soft drinks. CNS and cardiac effects may call for withdrawal of caffeine from diet.
- Clozapine -Possible increased clozapine plasma concentrations; increased risk for in adverse effects.
- Corticosteroids- Increased risk of tendinitis or tendon rupture, especially in patients older than 60 years.
- Cyclosporine –may cause additive nephrotoxic effects or interference with metabolism of cyclosporine.
- Didanosine- causes decreased absorption of ciprofloxacin.
- Histamine H2-receptor antagonists (cimetidine, ranitidine) – evidence of pharmacokinetic interaction has not been found.
- Iron preparations- Decreased absorption of ciprofloxacin. Administer ciprofloxacin tablets at least 2 hours before or 6 hours after ferrous sulfate and dietary supplements containing iron.
- Methotrexate- may cause increased methotrexate concentrations and increased risk of toxic effects.
- Metoclopramide-Increased rate of GI absorption of ciprofloxacin. Effect on ciprofloxacin bioavailability not clinically important.
- Multivitamins and mineral supplements- Decreased absorption of ciprofloxacin. Administer ciprofloxacin tablets, extended-release tablets, or oral suspension at least 2 hours before or 6 hours after supplements containing calcium, zinc, or iron.
- Omeprazole- Possible decreased concentrations and AUC of ciprofloxacin. Not considered clinically important.
- Phenytoin- Possible altered phenytoin concentrations. Use with caution.
- Probenecid- Decreased clearance of ciprofloxacin.
- Rifampin- In vitro evidence of indifferent against S. aureus; antagonism reported rarely
- Ropinirole- Increased ropinirole concentrations and AUC;-use with caution
- Sucralfate- Possible decreased GI absorption and decreased concentrations of ciprofloxacin; – Administer ciprofloxacin tablets, extended-release tablets, or oral suspension at least 2 hours before or 6 hours after sucralfate.
- Tizanidine-Increased risk of adverse effects. Concomitant use contraindicated.
- Theophyline-Possible increased theophylline concentrations and increased risk of theophylline-related adverse effects;-serious and fatal reactions reported;-If used concomitantly, closely monitor patient and theophylline concentrations and make appropriate theophylline dosage adjustments as needed, especially in geriatric patients.
Mode of delivery
Ciprofloxacin is available in preparations for both oral and intravenous administration. Topical preparations for eye and ear infections are also available. Ciprofloxacin is rapidly absorbed in the gastrointestinal tract. Its absorption is good though it is not completely absorbed. After absorption it undergoes a little first pass metabolism. Peak plasma concentrations are reached after half an hour to two and half hours. Its oral bioavailability in a fasting adult is approximately 50-85%. Following oral or intravenous administration it is distributed to many tissues of the body (Barry 1987).
Tissues that receive highest concentration of the drug include bile, cebrospinal fluid, lungs, kidney, cardiac tissue, endometrium, bone and cartilage. It is partially metabolized in the liver and excreted via the kidneys. Its elimination half life is 3 to 7 hours. Plasma protein binding is approximately 16% to 43%. There is an oral preparation which is an extended release preparation. There is a concerted effort by the researchers to make a ‘vehicle attached’ preparation. This preparation is expected to be implanted into infected bones where it will release bursts of the drug at a programmed rate. If it succeeds it will represent a major development in the history of this drug.
Acid-Base properties
The behavior of a drug in a solution greatly affects the pharmacokinetics and pharmacodynamics of the drug. An acid is a chemical compound that is capable of donating a proton and a base is capable of accepting a proton. When acid donates a proton it is converted to a base and when base accepts a proton it is converted the complimentary base. Ciprofloxacin contains multiple organic functional groups. Ciprofloxacin has a secondary alklyaminse group, two tertiary arylamines and a carboxylic acid (Stobberingh 1987). The two tertiary arylamines are weakly basic and are not significant in acid-base behavior of ciprofloxacin.
The carboxylic acid group is the proton donor. Therefore ciprofloxacin is an amphoteric substance because it has both basic and acidic properties. The pH of tissues determines whether ciprofloxacin donates a proton or accepts a proton. In an alkaline medium the carboxylic group yields a proton while in acidic tissue environment it accepts protons. In some cases both may proceed at the same time.
When a substance donates a proton or accepts a proton, it becomes ionized. Ionization alters the solubility of the substance. Depending on the pH of the tissue or solution, ciprofloxacin is expected to be more soluble in some circumstances.
Solubility of ciprofloxacin in water and other solvents
Ciprofloxacin is more soluble in water more than other solvents. Its solubility in water is more than that of other solvents by at least one unit (King 1984). As compared to other fluoroquinolones its solubility in water is influenced by temperature. The solubility of ciprofloxacin is enhanced when it is formulated as a hydrochloride (Kayser 1987). This is due to the fact that the hydrochloride group forms a Chloride ion. Its solubility in ethanol is lower than that of water at least one measured point.
Stability
Ciprofloxacin tablets are stable at temperatures less than 30 degrees Celsius for long periods of time. Extended release tablets should be stored at an optimum temperature of 25 degrees Celsius. Oral suspensions are stable at temperatures less than 25 degrees Celsius after reconstitution (Klinger 1985). Intravenous preparations are stable at between 5 to 25 degrees Celsius. Direct light and excessive heat can cause deterioration of this formulation. Infusions prepared using normal saline, 50% dextrose, 5% dextrose, and ringers lactate are stable for not more than 14 days. Refrigeration does not confer and longevity to this solution. Freezing is known to cause fluctuation in quality (Van Caekenberghe 1984).
History
Ciprofloxacin was discovered by Bayer a German drugs company in 1981 (Bayer Cipro 2008). A European patent was issued in 1982. In 1987, the FDA approved its use in the United States. The intravenous formulation was constituted in 1991. By 2001 proceedings in the United States of America had been initiated to explore the possibility of introducing a generic version of the drug. This would help reduce the cost of therapy with this drug significantly. The government of the United States of America has in the recent past negotiated with Bayer for the supply of the drug cheaply.
Controversial issues
Some people who suffered some adverse effects have sued the manufacturer of this drug. This toxicity has been referred to as fluoroquinolone toxicity. This represented a class suit instituted by both the consumers and an advocacy group. This litigation prompted the US government to act. In the recent past some legislation was passed that required the manufacturers to include warnings on the packaging. The warning was to include some common and severe adverse events. This may have slowed the sales in this market. Some clinicians have stopped prescribing this drug to particular groups of patients. They argue that resistance has already emerged and is no longer efficient. Some use cost as an explanation for their actions.
Conclusion
This paper looked at the pharmaceutical profile of ciprofloxacin in detail. Ciprofloxacin belongs to a class of synthetic drugs called fluoroquinolones. It was discovered in 1981 by Bayer, a German pharmaceutical company. It has found a lot of medical uses. It is the drug of choice for complicated bone infections. It is marketed around the world under different brand names. It is a relatively safe drug. Some adverse reactions associated with it are tendinitis, central nervous system toxicity, and visual disturbances.
References
Baddour et al (2005). AHA Scientific Statement: Infective Endocarditis. Circulation, 111: 3167-3184.
Barry, L., & Jones, N. (1987). In vitro activity of ciprofloxacin against gram-positive cocci. Am J Med, 82(4A):27-32.
Bayer. (2008). Cipro (ciprofloxacin hydrochloride). Tablets and Cipro (ciprofloxacin) oral suspension prescribing information. Web.
Hawkey, PM & Hawkey, CA. (1984). Comparative in-vitro activity of quinolone carboxylic acids against Proteeae. J Antimicrob Chemother. Nov;14(5):485-9.
Hirschhorn, L., & Neu, HC. (1987). In vitro activity of two new aryl-fluoroquinolone antimicrobial agents, difloxacin (A-56619) and A-56620 compared to that of other antimicrobial agents. Chemotherapy. 33(1), 1987.
Hoepelman et al. (1987). Comparative in vitro antimicrobial activity of carumonam (Ro 17-2301) and its influence on the activity of other antibiotics. Chemotherapy. 33(2):103-9.
Kayser, FH., & Novak J. (1987). In vitro activity of ciprofloxacin against gram-positive bacteria: an overview. Am J Med. 82(4A), 33-9.
King, A., Shannon, K., & Phillips, I. (1984). The in-vitro activity of ciprofloxacin compared with that of norfloxacin and nalidixic acid. J Antimicrob Chemother. 13, 325-31.
Klinger, JD., & Aronoff, SC. (1985). In-vitro activity of ciprofloxacin and other antibacterial agents against Pseudomonas aeruginosa and Pseudomonas cepacia from cystic fibrosis patients. J Antimicrob Chemother. 15 (6), 679-684.
Lagast, H., Husson, M., & Klastersky, J. (1985). Bactericidal activity of ciprofloxacin in serum and urine against Escherichia coli, Pseudomonas aeruginosa, Klebsiella pneumoniae, Staphylococcus aureus and Streptococcus faecalis. J Antimicrob Chemother.
Ligtvoet, E., & Wickerhoff-Minoggio, T. (1985). In-vitro activity of pefloxacin compared with six other quinolones. J Antimicrob Chemother. 16(4), 485-90.
Piddock et al. (1986). In vitro studies of S-25930 and S-25932, two new 4-quinolones. Eur J Clin Microbiol. 16 (3), 341-347.
Smith, SM., & Berman, E. (1986). The effect of ciprofloxacin on methicillin-resistant Staphylococcus aureus. J Antimicrob Chemother. 17(3), 287-95.
Stobberingh, E., Houben, W., & Van Boven, P (1987). In vitro evaluation of Ro 23-6240, a new fluorinated 4-quinolone. Chemotherapy. 33(3), 197-203.
Traub, WH., Spohr M., & Bauer, D. (1987). Gentamicin-and methicillin-resistant Staphylococcus aureus: in vitro susceptibility to antimicrobial drugs. Chemotherapy. 33(5), 361-75.
Turgeon, PL., Desrochers, C., & Mantha, R. (1987). Comparative in vitro activity of fluoroquinolones and other parenteral antimicrobial agents against urinary bacterial isolates and oxacillin-resistant. Staphylococcus aureus. Curr Ther Res Clin Exp. 31 (Supplement 2): S16-S23.
Van Caekenberghe, L., & Pattyn, R. (1984). In vitro activity of ciprofloxacin compared with those of other new fluorinated piperazinyl-substituted quinoline derivatives. Antimicrob Agents Chemother. 25:518-21.
Young, S., & Berlin, G. (1987). Inderlied CB. Activity of ciprofloxacin and other fluorinated quinolones against mycobacteria. Am J Med. 82(4A), 23-6.