Pharmacotherapeutics
Rivaroxaban is a type of medicine known as antithrombotic. It is normally used to make the blood flow easily through the vein. This medicine is used as the primary therapy for individuals who have deep vein thrombosis (Mayo Clinic, 2021). This is a condition that happens when a thrombus forms in one or more deep veins in an individual’s body, especially in the legs. As a result, this medicine is administered to prevent clotting. It can also be used in the treatment of pulmonary embolism.
The Drug Name and Its Therapeutic Category
The name of the drug is Rivaroxaban. It belongs to a therapeutic category called anticoagulants. These are medicines that can be used to treat and prevent blood clots in the body. Anticoagulation is defined as the primary therapy for DVT, and the primary goal of anticoagulant medication at the beginning of deep vein thrombosis treatment is to prevent thrombus extension to the lungs, as well as to prevent early recurrences of the disease (Mayo Clinic, 2021). Sometimes an anticoagulant medicine is used for a very short time period after a person has had heart surgery.
Pharmacodynamics
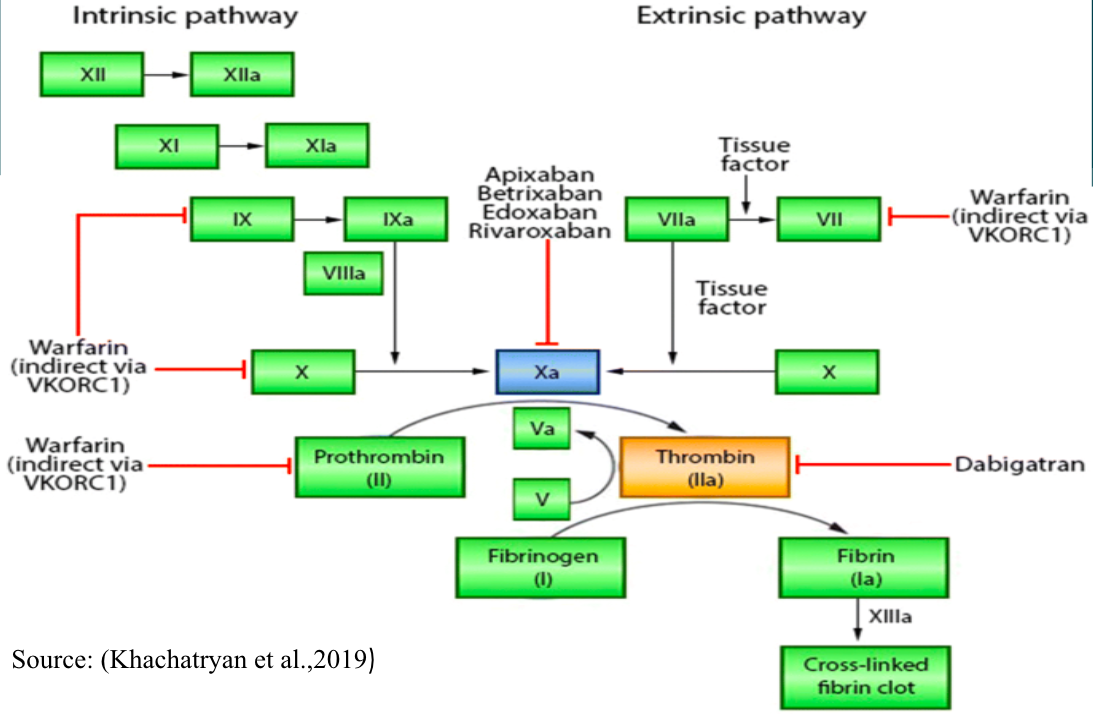
As shown in the figure, Rivaroxaban works by inhibitors factor Xa, which usually activates blood clotting. The clotting cascade is grouped into the intrinsic pathway, “internal injury,” and the extrinsic pathway, “external injury.” When an injury occurs, factor Xa goes on to activate fibrin which is responsible for clotting (Khachatryan et al., 2019). However, when Rivaroxaban is taken, as demonstrated above, it acts by selectively blocking the active site of factor Xa from performing its normal faction of activating fibrin in the platelets without requiring a co-factor.
Pharmacokinetics
Absorption
Rivaroxaban is usually absorbed through food. This explains why patients are advised to take it after eating. It takes about two-four hours for the medicine to be absorbed in the blood.
Distribution
Rivaroxaban uses plasma protein in the blood as a vehicle to move across the body.
Metabolized
Rivaroxaban is metabolized through oxidation and hydrolysis.
Excretion
The medicine is excreted out of the body via urine, renal discharges, and feces (Bratsos, 2019).
Dosage, Scheduling, Route, and Length of Therapy
Dosage
The medicine can be given in two dosing options. The first option is 15 mg, taken twice a day for 21 days after meals. The second option is 20 mg taken once every day.
Scheduling
The medicine can be taken once a day or twice daily, depending on its milligram.
Length of therapy
The treatment can take a period of not less than six months (Mayo Clinic, 2021).
Therapeutic Response
Response time
This is the time it takes for one to experience the action of a drug after intake. Rivaroxaban has a quick onset of action, and a patient should expect some action two to four after taking the drug.
Ways to enhance therapeutic response
A patient can also help in promoting the therapeutic response of the drug through non-drug measures. These measures include and are not limited to avoiding smoking, drinking a lot of water, engaging in exercise to increase blood flow, and avoiding sitting for a long period of time. In addition, eating a balanced diet can also help increase therapeutic response (Almarshad et al., 2018).
Side Effects, Adverse Effects, and Interactions
Side effects are undesirable secondary effect that happens together with desired therapeutic effect after taking Rivaroxaban. The effects include and are not limited to numbness, blood in coughs, the problem with breathing, the patient may experience instances of irritation, back pain, and traces of blood in the stool. A common side effect is bleeding, and it is advisable for a patient to immediately seek medical attention (Mayo Clinic, 2021). The side effect may cause health complications and should be monitored and averted.
Adverse effects are unexpected medical issues that occur in the course of treatment with a medicine. Some of the effects that a patient might experience when using Rivaroxaban include vertebral pain, bleeding, pain in the belly, faintness, and the presence of discharge in the wound (Mayo Clinic, 2021). For instance, a patient may experience excessive bleeding, especially in instances of small cuts. The symptom of a major adverse effect is a hematoma, and patients with this symptom are considered to encounter a life-threatening effect. They should seek medical attention with immediate effect because this can lead to mortality. However, a known effect experienced when using this medicine is hyperactivity, which is a state of being abnormally active. A patient should see a doctor when they experience hyperactivity.
A patient should know about drug-drug and drug-food interactions because of possible adverse effects. When taking Rivaroxaban, one should avoid drugs such as aspirin, mifepristone, ibuprofen, and naproxen (Vazquez, 2018). The interaction of these drugs with Rivaroxaban may cause intense bleeding. This means that a patient should stop taking the listed drugs once they are introduced to Rivaroxaban. However, currently, there is no evidence that limits a patient from taking any food when using Rivaroxaban.
Follow-Up Care
Follow-up care is integral for quick recovery and prevention of hospital readmission. When using Rivaroxaban, proper follow-up care is required because of the side effect such as bleeding and adverse effect like hyperactivity. A doctor should regularly communicate with the patient to determine whether they are following the prescription provided. (Mayo Clinic, 2021) For example, if the medicine was supposed to be taken twice a day after meals, a doctor should confirm compliance. In addition, one of the common side effects is bleeding. A patient should inform the doctor if they experience the mentioned side effects. In the event of dizziness, a patient should immediately call 911.
References
Almarshad, F., Alaklabi, A., Bakhsh, E., Pathan, A., & Almegren, M. (2018). Use of direct oral anticoagulants in daily practice. American journal of blood research, 8(4), 57.
Bratsos, S. (2019). Pharmacokinetic properties of rivaroxaban in healthy human subjects. Cureus, 11(8). Web.
Khachatryan, T., Hauschild, C., Hoff, J., Contractor, T., Khachatryan, A., Tran, H., Matsuo, B.,Jacobson, A. & Hilliard, A. (2019). Review of direct oral anticoagulants and guide for effective drug utilization. American Journal of Cardiovascular Drugs, 19(6), 525-539.
Mayo Clinic. (2021). Rivaroxaban (Oral Route). Mayo Foundation for Medical Education and Research. Web.
Vazquez, S. R. (2018). Drug-drug interactions in an era of multiple anticoagulants: a focus on clinically relevant drug interactions. Blood, The Journal of the American Society of Hematology, 132(21), 2230-2239. Web.