Introduction
Background Information
Enzymes are biological catalysts of a protein nature that are formed in living cells and have the ability to activate various chemical compounds. A characteristic of the activity of enzymes is the rate at which they catalyze a particular reaction. It is measured by the rate of substrate conversion or the rate of accumulation of reaction products.
Enzymes, like any proteins, are sensitive to the pH value of the substrate. The ionization of functional groups in the enzyme protein molecule depends on the concentration of hydrogen ions (H+). First of all, it is the hydrogen ions that act on the active site of the enzyme. At different pH values in the reaction substrate, the active site can be ionized either more weakly or more strongly, depending on the pH level. Secondly, the concentration of hydrogen ions affects the state of the protein part of the enzyme, determining the ratio of anionic and cationic groups in it, which affects the tertiary structure. It is during the formation of the tertiary structure of the enzyme that the active sites of the enzyme are formed. Thirdly, the pH of the substrate affects the degree of ionization of the substrate, enzyme-substrate complex, and reaction products. Thus, each enzyme has its own optimal pH value at which its activity is maximum.
Catalase is an enzyme that catalyzes the decomposition of hydrogen peroxide (H2O2) to molecular oxygen (O2) and water (H2O) and is involved in a number of redox reactions with various substrates. Catalase only works on substrates with pH levels between 7 and 11. If the pH is below 7 or above 11, the enzyme will denature and lose its structure. Catalase enzymatic activity can be observed by watching the amount of product appearing after a reaction, the product being oxygen bubbles. Control tests should also be used to verify the validity of the experiment.
Purpose
The purpose of this experiment is to determine how the pH level will affect the catalase activity. For this, catalase will be mixed with water with different levels of pH,, and hydrogen peroxide (H2O2) will be added. After that, the enzymatic activity will be determined by measuring the amount of oxygen bubbles produced.
Hypothesis
My hypothesis is that the enzymatic activity of catalase will rise up to a certain pH value, and after reaching it, it will decrease.
Method
Equipment Used
- I have prepared the following solutions:
- 5 ml of catalase;
- 20 ml of hydrogen peroxide (H2O2);
- 2 ml of water (H2O) adjusted to pH 3;
- 2 ml of water (H2O) adjusted to pH 5;
- 2 ml of water (H2O) adjusted to pH 7;
- 2 ml of water (H2O) adjusted to pH 9;
- 2 ml of water (H2O) adjusted to pH 11.
- Five clean test tubes, one for every reaction.
- Transfer pipets;
- A ruler to measure the amount of oxygen bubbles in the tubes in millimeters.
Collection of Data
Catalase activity produces oxygen bubbles as a result of the reaction. Thus, to assess this activity, the height of the bubble column in millimeters was measured with a ruler and written down in the form of a table.
Results
Data Table
Data Graph
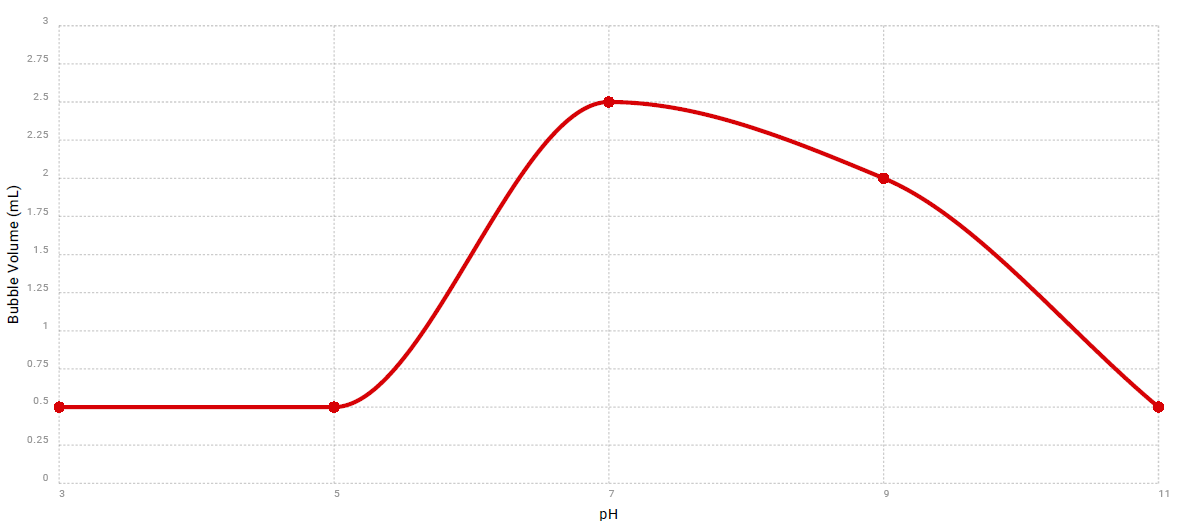
Description of Data
The data provided in the table shows how the enzymatic activity of catalase changes in different pH levels of substrate. The graph demonstrates visually how the catalase activity rose up to its highest point at pH 7, and then gradually dropped to almost inactive at pH 11.
Conclusion
Comparing Hypothesis with Results
The results of the experiment proved the correctness of the initial hypothesis. The activity of catalase was low at pH levels below 7, at 7 the enzyme was the most active, and then catalase was gradually losing its activity with a subsequent rise of pH up to 11.
Explanation
Catalase enzyme works best at pH 7, which is a neutral pH value. This indicates that it is the most active when there is neither excess acid nor base in the substrate. A change in pH to the acidic or alkaline side is accompanied by a more or less uniform drop in enzyme activity, as shown in the data graph.